Abstract
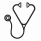
Gilteritinib (Gilt), a FLT3 tyrosine kinase inhibitor (TKI), is approved for the treatment of relapsed/refractory (R/R) FLT3-mutated (FLT3 +)acute myeloid leukemia (AML). However, long-term survival is limited by the development of drug resistance mutations in persistent FLT3 + clones. Combination regimens may deepen response and improve outcomes. Venetoclax (Ven), a BCL-2 inhibitor, is approved in combination with hypomethylating agents for newly diagnosed AML not suitable for standard induction therapy. FLT3 + AMLhas been associated with clinical resistance to Ven. Still, FLT3 TKIs + Ven have demonstrated synthetic lethality in preclinical models, prompting this multicenter, open-label, phase 1b trial (NCT03625505) to evaluate Ven + Gilt for R/R AML. Here, we report final response and survival endpoints, and molecular clearance among patients (pts) treated at the recommended phase two dose (RP2D).
The study design has been previously described (Daver, et al. ASH 2020, Abstract 333). Pts in the dose expansion cohort received Ven 400 mg + Gilt 120 mg (RP2D) daily in 28-day cycles, following Ven ramp-up. The primary endpoint was mCRc (complete response [CR] + CR with incomplete platelet recovery [CRp] + CR with incomplete blood count recovery [CRi] + morphologic leukemia-free state) to align with the ADMIRAL phase III trial (Perl, et al. NEJM 2019). Baseline co-mutations and serial FLT3 internal tandem duplications (ITD) allelic burden were assessed using the MyAML panel (Invivoscribe, San Diego, CA) and FLT3-ITD MRD assay with sensitivities of 5% and 0.001%, respectively.
As of the data cut off of January 31, 2021, 54 pts were treated at the RP2D. Fifty-two pts (as assessed locally) had FLT3 +AML; 41 had FLT3-ITD only, 8 had tyrosine kinase domain only, 3 had both mutations, and 2 were FLT3 wild type (wt). Additional baseline characteristics are in Table 1.
Grade 3 or 4 AEs occurred in 51 (94.4%) pts, and 39 (72.2%) pts had serious AEs. Grade 3 or 4 cytopenias occurred in 43 (79.6%) pts. AEs of special interest included tumor lysis syndrome (2 [3.7%]) and QT prolongation (1 [1.9%]). AEs leading to dosing interruptions (Ven, 27 [50%]; Gilt, 26 [48.1%]), reductions (Ven, 3 [5.6%]; Gilt, 4 [7.4%]), and discontinuation (Ven, 7 [13%]; Gilt, 7 [13%]) were reported for both study drugs. There was 1 treatment-emergent death of typhlitis.
Among FLT3 + pts with post-baseline assessments (51/52), mCRc was achieved by 38 pts (74.5%; CR/CRp/CRi, 19 [37.3%] pts), with a median follow-up time of 12 mo (range: 0.8 - 20.1). The mCRc in pts with prior TKI (32) or prior Ven (10) use were 78.1% and 60%, respectively. The mOS among all FLT3 +pts was 10 mo (95%CI: 6.6, NE), with a median DoR (mDoR) of 5.4 mo (95% CI: 3.3, 6.6). Pts with FLT3-ITD had a mOS of 10.5 mo (95%CI: 6.8, NE), and a mDoR of 5.6 mo (95% CI: 3.3, 8.3). For the 14 pts who had a transplant, the mOS was not reached (95%CI: 10.0, NE) vs 6.89 mo (95%CI: 3.0, 10.5) for those who did not have a transplant (37). The mOS for pts with prior TKI or Ven was 9.6 (95% CI: 4.3, NE) and 10.5 mo (95% CI: 2.0, NE), respectively, with a mDoR of 9.6 (95% CI: 4.3, NE) and 6.2 mo (95% CI: 2.6, NE), respectively. The 60-day mortality rate was 11.8% (95% CI: 4.4, 23.9).
The mCRc for NPM1 + and NPM1wt were 92.3% and 61.1%, respectively. mCRc for DNMT3A + and DMNT3A wt pts were 82.4% and 64.3%, respectively. WT1 + and WT1 wt mCRc were 66.7% and 77.3%, respectively. The mCRc for NPM1 + and DNMT3A + co-mutated pts was 100%. In a post hoc analysis of the 30 analyzable mCRc pts with at least one follow-up MRD assessment, 17 (56.7%) achieved molecular clearance defined as FLT3 allelic burden < 10 -2. The mOS of pts achieving mCRc with FLT3 allelic burden of < 10 -2 vs mCRc with FLT3 allelic burden ≥ 10 -2 (Figure 1) was not reached (95% CI: 9.76, NE) vs 6.83 mo (4.21, NE).
Ven + Gilt achieved high mCRc in patients with R/R FLT3 + AML, with or without prior TKI exposure, and an encouraging mOS. FLT3 mutation clearance was seen in a majority of patients and associated with longer OS. Encouraging remission rates were observed across many genotypes and were particularly high among pts with NPM1 + +/- DNMT3A co-mutation. Cytopenias were common but manageable with appropriate Ven or Gilt dosing modifications. Serial NGS molecular data, as well as updated survival data will be presented at the meeting.
Daver: Hanmi: Research Funding; Abbvie: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Novimmune: Research Funding; FATE Therapeutics: Research Funding; Glycomimetics: Research Funding; Amgen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Perl: Actinium: Consultancy; Genentech: Consultancy; Roche: Consultancy; Astellas: Consultancy, Research Funding; Forma: Consultancy; Syndax: Consultancy; Loxo: Consultancy; Fujifilm: Research Funding; Arog: Research Funding; BMS/Celgene: Consultancy; AbbVie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Onconova: Consultancy; Sumitomo Dainippon: Consultancy. Levis: Amgen, Astellas Pharma, Daiichi-Sankyo, FujiFilm, and Menarini: Honoraria; Astellas and FujiFilm: Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Ritchie: Astellas: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Other: travel support, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; NS Pharma: Research Funding; Incyte: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria; Jazz: Consultancy, Research Funding; Protaganist: Consultancy, Honoraria; ARIAD Pharmaceuticals: Ended employment in the past 24 months, Speakers Bureau; Pfizer: Consultancy, Research Funding. Litzow: Astellas: Research Funding; Omeros: Other: Advisory Board; Pluristem: Research Funding; AbbVie: Research Funding; Amgen: Research Funding; Actinium: Research Funding; Jazz: Other: Advisory Board; Biosight: Other: Data monitoring committee. McCloskey: Jazz: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Smith: AbbVie: Research Funding; Revolutions Medicine: Research Funding; Daiichi Sankyo: Consultancy; FUJIFILM: Research Funding; Astellas Pharma: Consultancy, Research Funding; Amgen: Honoraria. Schiller: Takeda: Research Funding; Trovagene: Research Funding; Tolero: Research Funding; Ono: Consultancy; Novartis: Consultancy, Research Funding; ASH foundation: Other: Chair-unpaid; Pfizer: Current equity holder in publicly-traded company, Research Funding; Incyte: Consultancy; Sanofi: Honoraria, Research Funding, Speakers Bureau; Ariad: Research Funding; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Ono-UK: Consultancy, Research Funding; Karyopharm: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Onconova: Research Funding; Mateon: Research Funding; Sangamo: Research Funding; Samus: Research Funding; Regimmune: Research Funding; PrECOG: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Kaiser Permanente: Consultancy; Cyclacel: Research Funding; MedImmune: Research Funding; Ambit: Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; Elevate: Research Funding; Bio: Research Funding; Pharma: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Biomed Valley Discoveries: Research Funding; Eli Lilly: Research Funding; Sellas: Research Funding; Geron: Research Funding; Genentech-Roche: Research Funding; Gamida Cell Ltd.: Research Funding; FujiFilm: Research Funding; Forma: Research Funding; Delta-Fly: Research Funding; Deciphera: Research Funding; Daiichi-Sankyo: Research Funding; Constellation Pharmaceuticals: Research Funding; Celator: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Astellas: Honoraria, Research Funding, Speakers Bureau; Arog: Research Funding; Actuate: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Abbvie: Research Funding; Leukemia & Lymphoma Society: Research Funding; Bluebird Bio: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Bradley: AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Tiu: Astellas Pharma: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Naqvi: Genentech/Roche: Current Employment, Current holder of stock options in a privately-held company. Dail: Genentech/Roche: Current Employment, Current equity holder in publicly-traded company. Siddani: AbbVie: Current Employment, Current holder of stock options in a privately-held company. Wang: AbbVie: Current Employment, Current holder of stock options in a privately-held company. Chyla: AbbVie: Current Employment, Current equity holder in publicly-traded company. Lee: AbbVie: Current Employment, Current holder of stock options in a privately-held company. Altman: Astellas: Consultancy, Other: Advisory Board, Research Funding; AbbVie: Consultancy, Other: Advisory Board, Research Funding; Biosight: Consultancy, Other: Travel fees, Research Funding; Daiichi Sankyo: Consultancy; Kura Oncology: Consultancy; Syros: Consultancy; Theradex: Consultancy, Other: Advisory boards; GlycoMimetics: Other: Participation on an advisory board; ALZ Oncology: Research Funding; Amgen: Research Funding; Aprea: Research Funding; BMS: Research Funding; Boehringer Ingelheim: Research Funding; Fujifilm: Research Funding; Immunogen: Research Funding; Kartos: Research Funding; Kura: Research Funding.
Venetoclax is a B-cell lymphoma 2 inhibitor approved in combination with azacitidine, or decitabine, or low-dose cytarabine for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. Gilteritinib is a kinase inhibitor indicated for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation as detected by an FDA-approved test.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract